In order to do some genome sequencing on C.bairid and Hematodinium, we need hihg molecular weight gDNA. I attempted this twice before, using two different methods (Quick DNA/RNA Microprep Kit (ZymoResearch) on 20200122 and the E.Z.N.A Mollusc DNA Kit (Omega) on 20200108) using ~10yr old ethanol-preserved tissue provided by Pam Jensen. Both methods yielded highly degrade gDNA. So, I’m now attempting to get higher quality gDNA from the RNAlater-preserved hemolymph pellets from this experiment.
Used the Quick DNA/RNA Microprep Kit (ZymoResearch) to perform four parallel isolations using four separate aliquots of sample 6129_403_26 with the following changes/notes:
Used 75uL of each hemolyph pellet slurry from each tube
Eluted all columns with a single 30uL elution (i.e. used 30uL for first column, then used that same elution to elute the next column, etc.) to try to concentrate the sample.
DNA was quantified using the Roberts Lab Qubit 3.0, using 1uL of sample, with the ds DNA BR Assay (Invitrogen).
Used 4uL of sample (~200ng) to run on 1x low-TAE 0.8% agarose gel.
RESULTS
UPDATE: I isolated additional DNA later in the day and combined this isolation with the one from later. See this post. I’ll leave the original info below for posterity.
Qubit results (Google Sheet):
-
[RNA] = 58.4ng/uL
Yield: 1752ng
Yields a bit lower than I would’ve liked, but this is isolation is primarily to see what the gDNA integrity looks like from these RNAlater-preserved hemolyph samples.
Sample was stored at -80oC in:
Rack 15, 4, 5 in C.bairdi gDNA Box #2
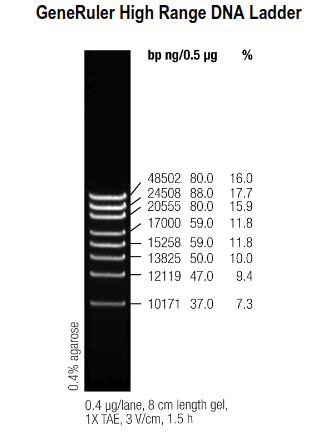
GeneRuler HighRange Ladder (ThermoFisher):
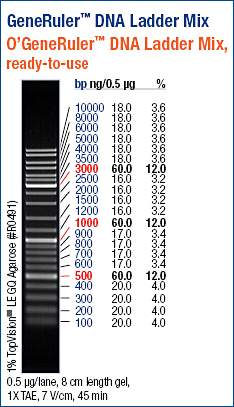
GeneRuler DNA Ladder Mix (ThermoFisher):
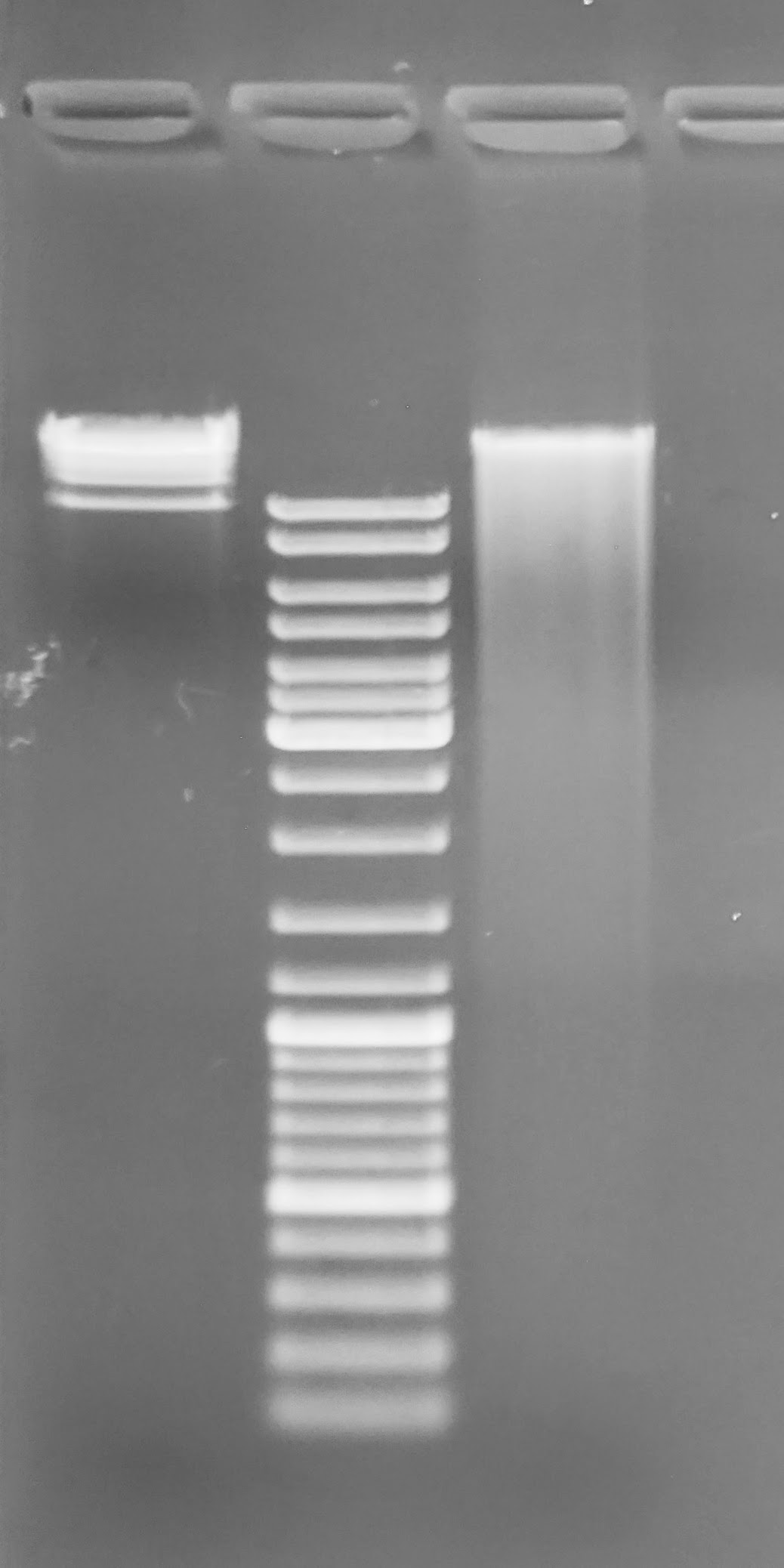
Alrighty, this gDNA looks significantly better than the two previous attempts using the EtOH-preserved tissue. Still smearing, but the bulk of the sample is high molecular weight gDNA, which will be suitable for sequencing via PacBio and/or NanoPore. Will isolate some more DNA in order to get enough to send for PacBio sequencing (need ~4x this amount…).