I ran this PCR a couple of times before and, embarrassingly, I had ordered/used the wrong primers.
Well, I ordered the correct universal cytochrome oxidase primers and used those!
| SR ID | Primer Name | Sequence |
|---|---|---|
| 1739 | HC02198 | taaacttcagggtgaccaaaaaatca |
| 1738 | LCO1490 | ggtcaacaaatcataaagatattgg |
| 1736 | COCsi546r | AAGTAACCTTAATAGATCAGGGAACC |
| 1735 | COCgi269r | TCGAGGAAATTGCATGTCTGCTACAA |
Primers and cycling parameters were taken from this publication:
Universal cytochrome oxidase primers were from this paper:
This is a multiplex PCR, where the HC02198 and LCO1490 primers should amplify any Crassostrea spp. DNA (i.e. a positive control - 697bp) and the other two primers will amplify either C.gigas (Cgi269r - 269bp) or C.sikamea (Csi546r - 546bp).
Master mix calcs:
| Component | Single Rxn Vol. (uL) | Num. Rxns | Total Volumes (uL) |
|---|---|---|---|
| DNA | 4 | NA | NA |
| 2x Apex Master Mix | 12.5 | 18 | 225 |
| HC02198 (100uM) | 0.15 | 18 | 2.7 |
| LCO1490 (100uM) | 0.15 | 18 | 2.7 |
| COCgi269r (100uM) | 0.1 | 18 | 1.8 |
| COCsi546r (100uM) | 0.1 | 18 | 1.8 |
| H2O | 8 | 18 | 144 |
| 25 | Add 21uL to each PCR tube |
Cycling params:
95oC for 10mins
30 cycles of:
- 95oC 1min
- 51oC 1min
- 72oC 1min
72oC 10mins
PCR reactions were run on a 1.5% agarose, 1x low TAE gel with ethidium bromide.
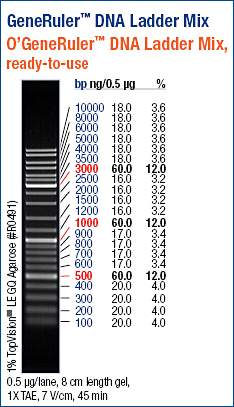
Used the GeneRuler DNA Ladder Mix (ThermoFisher) for all gels:
RESULTS
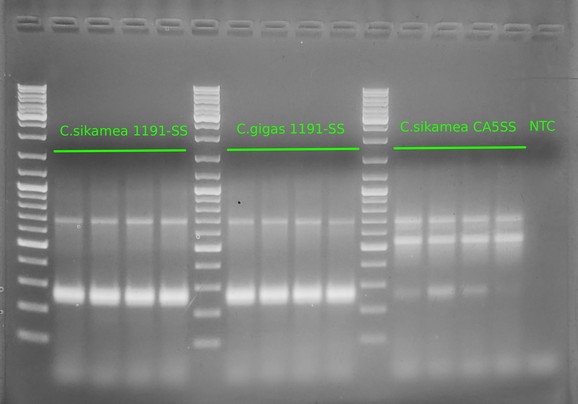
Alrighty, this is what we expected to see (at least in terms of primer functionality). We see:
A band of ~700bp in all samples (i.e. the universal cytochrome oxidase primers)
A band of ~270bp in the
C.gigas 1911SSsamples
Now, this is where things get interesting…
In the C.sikamea CA5SS:
A prominent band of ~550bp, indicating these are C.sikamea
A less prominent, but definitive, band at ~270bp, indicating the presence of C.gigas cytochrome oxidase sequence. Suggests that this particular stock has hybridized with C.gigas (or, there was some sort of cross contamination of DNA/tissue; unlikely as we don’t see potential cross contamination of C.sikamea DNA in the other samples).
In the C.sikamea 1911SS:
- A prominent band of ~270bp, indicating these are C.gigas, despite the bag label indicating them as Kumamoto and/or Pacific oysters (see image below)
